What is Ozone?
Ozone generated from three oxygen atoms,which is why ozone is also known as trioxygen in science. It is a volatile molecule that gradually decomposes to oxygen at room temperature. Specific gravity is 1.54 times that of air and three times that of oxygen, and its solubility in water is about ten times that of oxygen.
Ozone has a wide range of effects, including sterilization, deodorization, decolorization, and oxidation, and its oxidizing power is second only to fluorine in the natural world.
Ozone is a light blue gas,when the concentration in the air is low,it will occour fresh air,but after thunderstorms and lightning,it will produce a sharp smell due to the high ozone concentration.
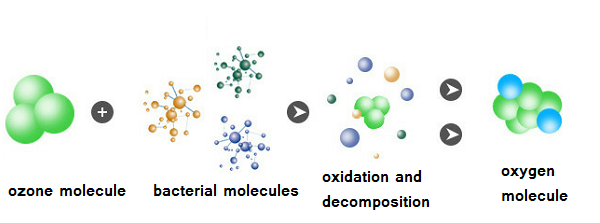
Ozone sterilization principle: the sterilization process is a biochemical oxidation reaction. Ozone can directly oxidize bacterial cells, that is, destroy their DNA,thereby achieving an inhibitory effect. It also has a damaging effect on viral RNA; it has a certain oxidizing effect on various toxins and reduces their toxicity.
Ozone is a Nature's Powerful Guardian.
Ozone is not only the major component of nature,but also an improtant element as a protector of the earth.It mainly exists in the earth's stratosphere, forming the ozone layer, which effectively absorbs most of the harmful ultraviolet rays from the sun and protects the earth.And protect organisms from their damage.
Ozone,also known as Enhanced Oxygen,it reacts chemically with many organic compounds,and through chemical reactions,ozone can be applied flexibly and pragmatically in a variety of conditions and locations.
Ozone,the powerful nature molecule.Act as a protector for the earth ecology and play an important role on our daily lives.With the advancement of ozone technology, the application of ozone will continue to expand, bringing more benefits to our lives and environmental protection.
OZONE is 100% NATURAL ,PURE ELEMENT
Getting more information about ozone:https://www.britannica.com/science/ozone
As we mentioned above,what other properties does ozone have
| Property | Ozone |
| Symbo | Oӡ |
| Molecular Weight | 48 g/mol |
| Densitivity | 1.46 g/cm³ |
| Solubility | 570 mg/L(20℃) |
| Melting point | −192.7℃ |
| Boiling Point | −111.9℃ |
| Appearance | Blue gas;dark blue fluid |
| Flammability | None |
